U.S. News: Digital Media Is ‘Like Cocaine’ for Babies & Developing Brains
Credit: U.S. News By: Dr. Leah Light November 7, 2018 Click here to read

Credit: U.S. News By: Dr. Leah Light November 7, 2018 Click here to read

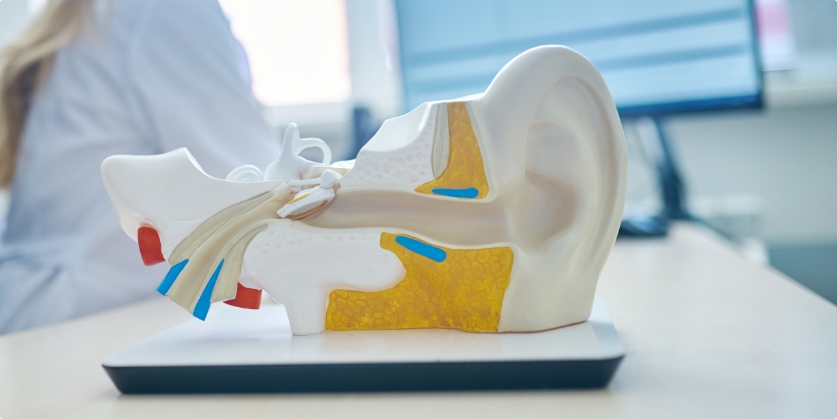
One of the most well known neuroaudiologist researcher is Nina Kraus, PhD.

Facebook. Why is it so popular? My husband dislikes social media. He